Kelvin–Planck and Clausius Statement
The violation of the Kelvin–Plank Statement by Violating the Clausius Statement can be understood through a hypothetical scenario illustrated in Figure 1.
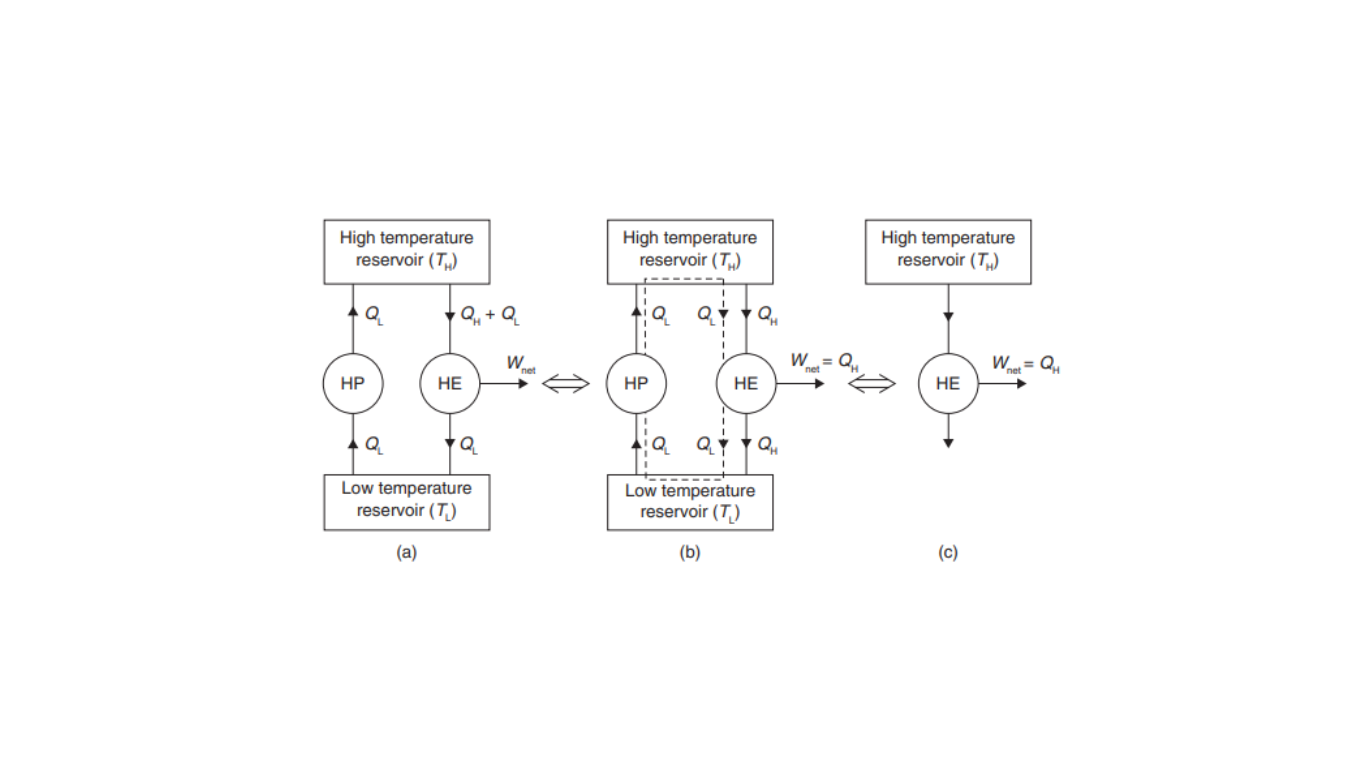
In this scenario, let’s assume a heat pump (a device that transfers heat from a low-temperature reservoir at TL to a high-temperature sink at TH) operates in a manner that appears to violate the Clausius statement. It accomplishes this by receiving heat, QL, from the low-temperature reservoir without the input of any external work. Simultaneously, a heat engine (another device) is supplied with a larger quantity of heat, (QH + QL), from a high-temperature source at TH. The heat engine produces a net work output, Wnet, equal to QH, and rejects heat, QL, to the low-temperature reservoir. When these two devices are considered together, as shown in Figure 1 (b), it becomes apparent that the heat pump helps facilitate the flow of heat QL from the low-temperature reservoir to the high-temperature reservoir. Conversely, the heat engine essentially supplies back the heat QL from the high-temperature reservoir to the low-temperature thermal energy reservoir.
Now, when we analyze the equivalent system in Figure 1.(c), we can see that a heat engine is receiving heat from a single reservoir and producing an equivalent amount of work. This equivalent system effectively operates as a perpetual motion machine of the second kind (PMM-2). Such a machine violates the Kelvin–Plank Statement, which is a fundamental principle of the second law of thermodynamics.
This scenario demonstrates that violating the Clausius Statement, which deals with the direction of heat flow, ultimately leads to the violation of the Kelvin–Plank Statement. Therefore, it can be concluded that the two statements, Kelvin–Plank and Clausius, are equivalent in their implications regarding the limitations on the efficiency and operation of heat engines and refrigeration systems.