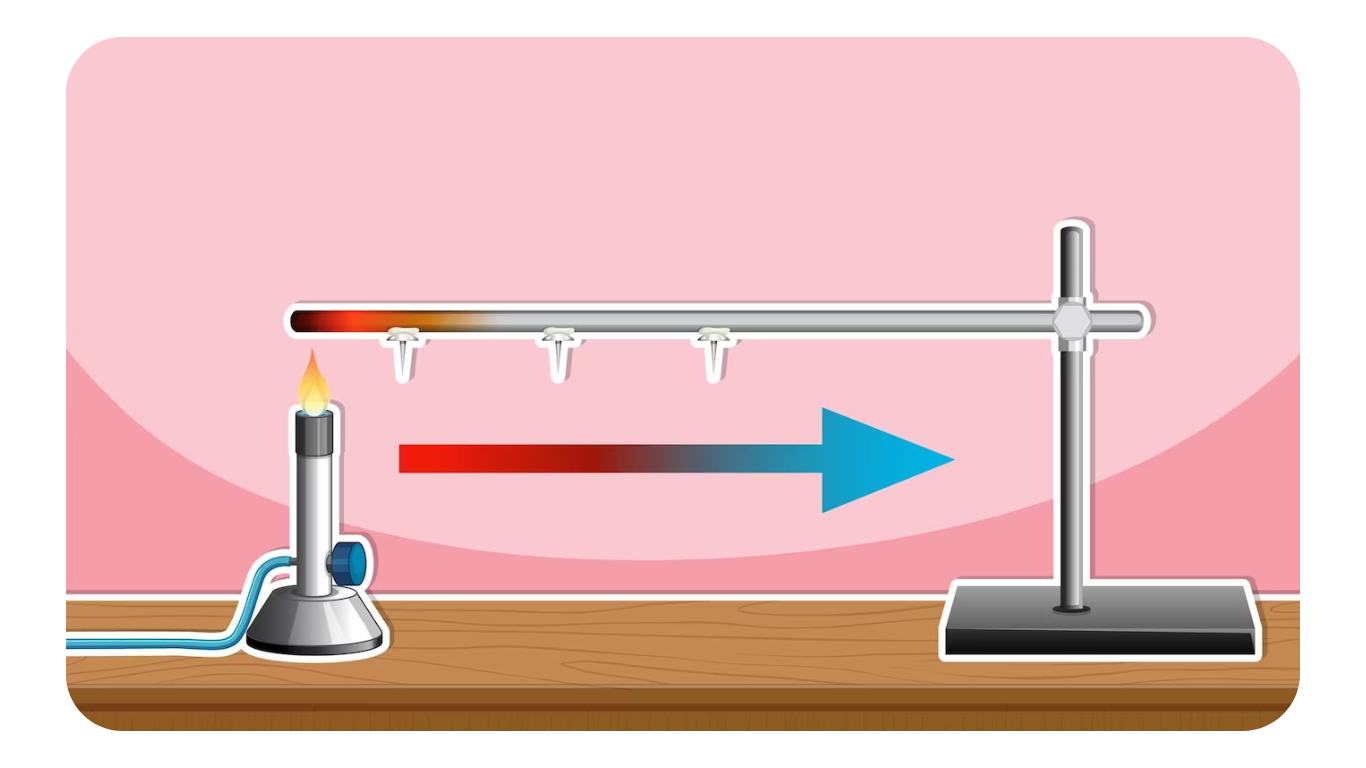
A system achieves thermodynamic equilibrium when it fulfills the requirements of various types of equilibrium, namely mechanical equilibrium, thermal equilibrium, chemical equilibrium, and electrical equilibrium.
Mechanical Equilibrium
A system attains mechanical equilibrium when there are no unbalanced forces acting on any parts of the system or the system as a whole. To be in mechanical equilibrium, there should be no pressure gradient present within the system.
Thermal Equilibrium
Thermal equilibrium is achieved when there is no temperature difference between different parts of the system or between the system and its surroundings.
Chemical Equilibrium
Chemical equilibrium is attained when there are no ongoing chemical reactions within the system, and there is no movement of any chemical constituents from one part of the system to another.
Electrical Equilibrium
Electrical equilibrium is reached when there is no electrical potential gradient present on any parts of the system or the system as a whole.
When a system satisfies all the conditions of mechanical, thermal, chemical, and electrical equilibrium, it is considered to be in a state of thermodynamic equilibrium.